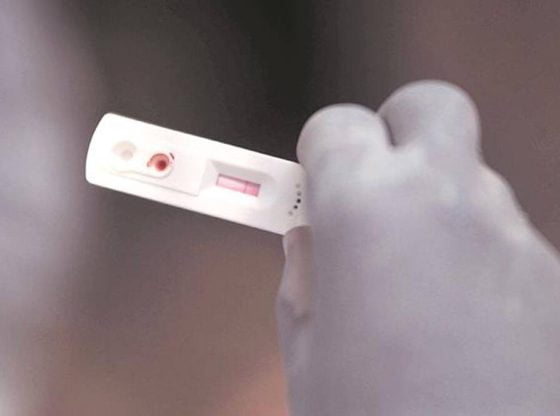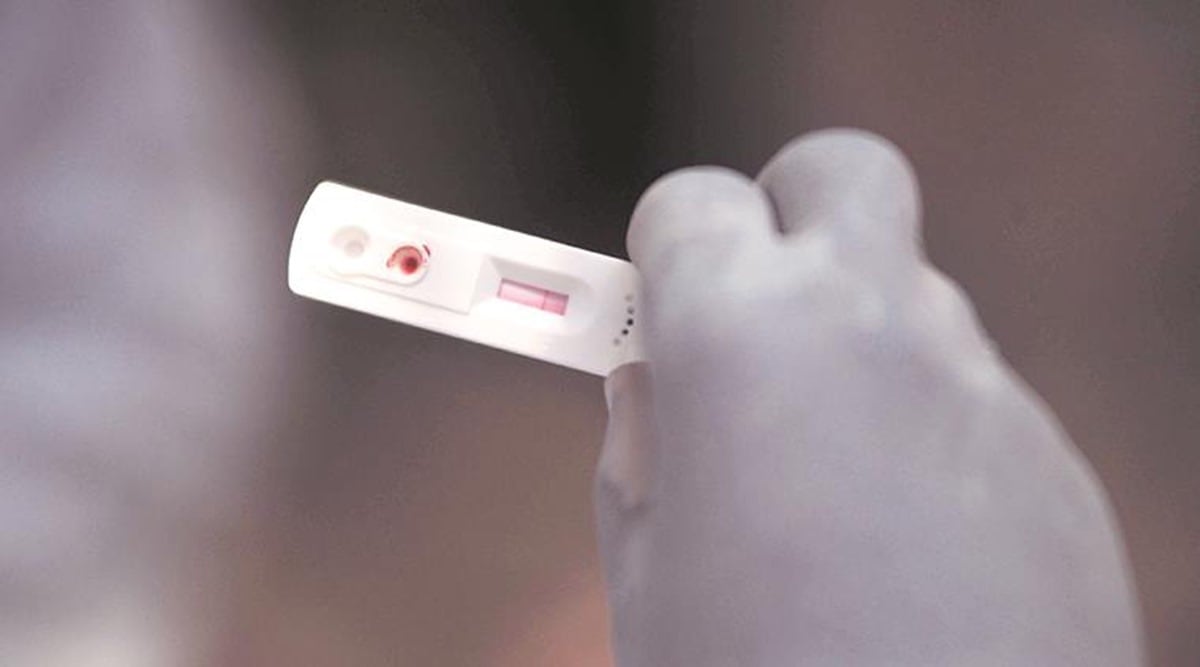
After the Pune division of the Food and Drugs Authority (FDA) asked chemists to keep a record of people buying home antigen test kits to ascertain Covid-19 infection, the commissioner of Brihanmumbai Municipal Corporation (BMC) Thursday issued similar orders and asked chemists and others to share the data with the FDA and the BMC’s Epidemiology Cell via email every day in the evening.
This comes amid a surge in the number of Covid cases and rise in cases of the Omicron variant, many in Mumbai are reportedly purchasing home testing kits.
The circular issued by municipal commissioner I S Chahal Thursday stated that all manufactures, distributors, chemists, dispensaries should maintain records of sale of home testing antigen kits.
According to the guidelines, chemists, pharmacies, dispensaries and medical stores have been given a form in which they must fill in details, including the name, address, mobile number and quantity of home testing kits purchased by an individual. BMC will take further action on the data shared with them in the prescribed format.
The guidelines are based on the central and state government’s recent directives to maintain record of home antigen test kits.
After it was found that in most of the cases patients did not upload the details of their tests on the ICMR web portal and there was no tracking of such patients who could be potential virus spreaders, BMC said its ward teams will track buyers and ensure the results from the home tests are uploaded on the website or the mobile app.
Chahal’s order further said, “It is expected that all Covid positive tests through rapid antigen test kits or home tests kits will be reported to ICMR by concerned laboratory/ individual through mobile app. However, it seems that, there may be some cases which has been tested on home testing kits but not reported in ICMR resulting into no tracking of such patients and the infection keeps spreading to their contacts, therefore, it is necessary to keep a track on such person/patients to contain the spread of virus.”
The guidelines have fixed roles of distributors, manufactures, suppliers, chemists, medical stores and dispensaries to keep a record of the kits and also inform buyers to report the test results on the given app.
[“source=indianexpress”]